Abstract
Introduction: Acute lymphoblastic leukemia (ALL) is the most common cancer affecting children and its cure rates are close to 90%. Nevertheless, relapsed ALL is still a major cause of cancer-associated deaths in children. Therefore, the multicenter trial Acute Lymphoblastic Leukemia-Relapse Study of the Berlin-Frankfurt-Münster Group (ALL-REZ BFM) 2002 was designed to improve the prognosis of children with relapsed ALL by introducing new chemotherapy elements. Little is known though about the prognostic relevance of deviations from the ALL protocols. This study aimed to investigate the characteristics of such protocol deviations and to determine their prognostic relevance for the relapsed disease.
Methods: We performed a retrospective analysis of 686 children and adolescents up to 18 years of age with first relapse of ALL who were enrolled between 2002 and 2012 in the ALL-REZ BFM 2002 Study. Deviations were identified in terms of time and type. In order to investigate the prognostic importance of the time delay, the 90th percentile of delays between defined treatment elements (i.e. 15.5 days) was used as a cut-off point. Furthermore, deviations were categorized as avoidable (logistics, family/PI decisions) and non-avoidable (clear medical indication).
The protocol deviation occurred during the course of therapy was therefore considered as a time-dependent variable. Disease-free survival, (DFS, induction failures excluded) and OS were analyzed using Kaplan-Meier estimate, log-rank test, Mantel-Byar test and Cox/logistic regression (univariate and multivariate analyses).
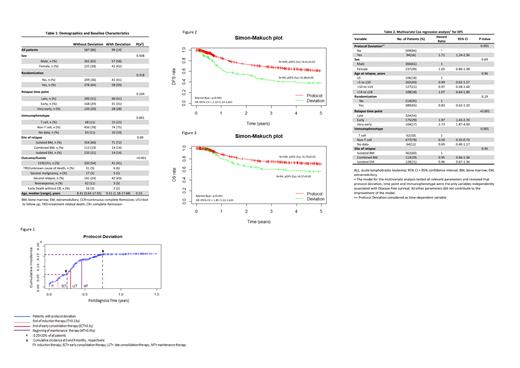
Results: A total of 587 patients (86%) received the protocol therapy, whereas 99 patients (14%) underwent deviations during their treatment. Baseline characteristics were compared in both groups (Table 1). Deviations were categorized as: change of chemotherapy in 48% of the patients (n=47/99), stop of chemotherapy in 10 % (n=10/99), non-protocol compliant reaction to MRD findings in 28% (n=28/99) and change of radiation treatment in 14% (n=14/99).
The cumulative incidence of protocol deviation was 6% and 15.5% at 3 and 9 months, respectively (Figure 1). The remission rate of patients with protocol deviation during induction was slightly lower compared to patients without such deviation (24/29, 83% vs. 579/657, 88%; P=0.38). The estimated 5-year probabilities of DFS and OS between the patients treated according to protocol therapy and those with deviations were significantly different (pDFS, Protocol therapy: 0.61 ± 0.02 vs. Deviation: 0.38 ± 0.05, P < 0.001; pOS, Protocol: 0.70 ± 0.02 vs. Deviation: 0.57 ± 0.05, P < 0.001; Figure 2, 3).
In multivariate analyses protocol deviation, time point of relapse and immunophenotype turned out to be independent prognostic factors of the DFS (Table2). The hazard ratio of the protocol deviation was significant only in patients with the late relapse, whereas in patients with early/very early relapse no significant prognostic effect could be found [late (n=40): HR=2.53, 95%CI 1.53-4.21, P<0.001; early (n=29): HR=1.49, 95%CI 0.88-2.51, P=0.13; very early (n=25): HR=1.50, 95%CI 0.84-2.66, P=0.17].
Moreover, DFS probabilities were comparable for avoidable (n=63) and non-avoidable (n=31) deviations (0.43 ± 0.07 vs. 0.40 ± 0.09, P=0.9; Non avoidable: HR=1.19, 95%CI=0.68-2.10, P= 0.53). In terms of the time-related deviations, the extent of the delays between induction and beginning of consolidation therapy was not significantly associated with the DFS [≤90th percentile (n=319/603); pDFS: =0.60 ± 0.02 vs. >90th percentile (n=36/603); pDFS: 0.56 ± 0.08, P=0.3]. Treatment delays during other phases of the protocol were also not related to the outcome.
Conclusion: While protocol deviations did not significantly affect remission rates in this study, they were significantly related to inferior DFS. Since a relevant part of the protocol deviations could be classified as avoidable, strict protocol compliance should lead to improved outcomes. The prognostic impact of protocol deviations was in particular relevant in patients with late relapse with generally rather chemosensitive diseases and better prognosis. In this analysis, treatment delays did not influence the outcome.
von Stackelberg: Amgen: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Jazz: Consultancy, Honoraria; Miltenyi: Consultancy, Honoraria.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal